Blood Sample Artifacts
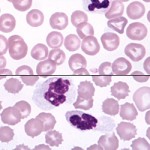
Blood Sample: Fresh vs Overnight
Blood sample artifacts are errors that can occur during the collection and handling of a sample. This results in damage and incorrect interpretation. Blood sample artifacts can be caused by poor technique, a sample aging, or poor maintenance of the staining solutions.
Collection Artifacts
- Difficult venipuncture
- Slow or traumatic venipuncture such as poking for the vein and exiting the vein during sample withdrawal can lead to platelet clumping and can even cause small microclots in the sample or worse: clotting of the sample.
- For AIHA dogs difficult venipuncture can shear red blood cells, called artifactual hemolysis. This can affect cell counts and can even seem to indicate intravascular hemolysis.
- Low sample volume
- Collecting a small blood volume and putting it into a standard 5 mL EDTA tube will cause shrinkage of red blood cells. This causes an false decrease in the mean cell volume (MCV) and false increase in mean cell hemoglobin concentration (MCHC) of red blood cells.
- Inappropriate mixing with anticoagulant
- A blood sample should be mixed well with anticoagulant during and right after collection using gentle inversions. Inadequate mixing can result in clotting of the sample (microclots).
- Dilution with fluids
- Collecting blood through a catheter is not the best practice. The catheter should be flushed with sterile saline and the first 3 mL of blood should be discarded to prevent diluting the sample.
- Rough handling
- Blood tubes should not be shaken. Blood should not be forced through needles, nor should blood be vigorously pushed into tubes. Mishandling like this can cause shearing of red blood cells (hemolysis) and platelet clumping. Samples should be handled very carefully.
Sample Storage Artifacts (age-related)
- Red blood cells
- Lysis can lead to a decrease in the RBC count and HCT.This can show falsely high values for MCH and MCHC.
-
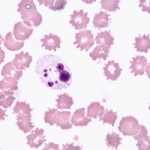
Crenation Crenation is a red blood cell that is “spiculated.” Also called a burr cell.
- Red blood cells will swell with water when stored too long. This will cause a falsely increased mean cell volume (MCV) and decreased mean cell hemoglobin concentration (MCHC). HCT is dependent on the MCV so the HCT may be higher than normal.
- White blood cells
- Age related swelling can mimicking band neutrophil formation.
- Prolonged storage can lead to Döhle bodies and this mimics toxic changes.
- With storage leukocytes can begin to look like and be misinterpreted as nucleated red blood cells (reticulocytes.)
- These changes can decrease a white cell count (lysed cells are not counted) and can affect the accuracy of a differential cell count. Sometimes, the changes are so severe, that an accurate differential cell count cannot be performed.
- Platelets
- Clumping of platelet decreases the platelet count and increases the mean platelet volume (MPV) A small clump of platelets can appear to be a single large platelet. Large platelet clumps are excluded from the count altogether.
- Degranulation makes platelets difficult to see and count.
Stain Artifacts
- Stains should be tightly capped when not in use
- Do not top-off the solutions
- Water artifact
-
Water Artifact Water in the fixative can cause a severe moth-eaten appearance to the cell. It usually occurs when using improperly stored stains. This can cause a refraction error or artifact when focusing up and down on a cell in a microscope, This artifact will flash or it may appear dark or it could even look bright. It can be mistaken for inclusions in the red blood cell like a parasite.
-
- Stain precipitate
- Old staining solutions and poor rinsing of slides after using stains can leave smears that block view of the sample or may mimic parasites or bacteria.
Anticoagulants
- EDTA
- This anticoagulant preserves cells and prevents clotting by chelating calcium. Binding of antibodies in the plasma to platelets or red blood cells can occur however.
- Antibodies binding to platelets causes them to clump and this leads to a falsely decreased platelet count.
- Antibodies binding to red blood cells causes them to aggregate. This mimics agglutination and that is a key feature in red blood cells that is diagnostic for immune-mediated hemolytic anemia. It is really an artifact but it can lead to an mistaken diagnosis of AIHA IMHA. This binding of EDTA antibodies to platelets and red blood cells can be reduced by collecting blood into citrate anticoagulant, however this should not be done routinely.
- Citrate
- Citrate anticoagulant also chelates calcium, but is a gentler chelator than EDTA. Calcium can be added back to the tube to perform blood testing. Counts will be lower by at least 10% compared to blood collected into EDTA.
- Canine platelet counts are still underestimated in citrate samples compared to EDTA samples, even taking into account the 10% dilution effect. Platelets will appear larger and less granulated in citrate suggesting that they were activated.
- Heparin
- Heparin is not recommended for hematologic testing. Platelets and leukocytes frequently clump in heparin, causing erroneous counts (absolute counts and a differential leukocyte count). Heparin also introduces a staining artifact (smears look a garish pink-purple) which affects blood smear examination.
Sample collection and handling guidelines
Samples in storage and for shipping should be kept at refrigerator temperatures, not frozen. This helps reduce changes in cells that can occur with storage. Prevent direct contact of the tube with ice, this can cause freezing and lysis of red and white blood cells. Blood smears should be made from freshly collected blood. Blood in tubes should be kept cold while slides should be stored and shipped at room temperature. Both tubes and slides should be placed in break-proof containers for shipping.
« Back to Glossary Index